Abstract
Background Improvements in Magnetic Resonance Imaging for evaluation of iron loading, alongside access to orally bioavailable iron chelators, have led to improvements in life expectancy and reproductive potential for patients with beta Thalassemia Major (TM) and Thalassemia Intermedia (TI). However, pregnancy in these individuals has been associated with adverse maternal and neonatal outcomes, the frequency of which is not well-delineated. The objective of this systematic review is to provide risk estimates of the maternal and neonatal outcomes in TM and TI, as well as explore the impact of pregnancy on indices of iron status.
Study Design and Methods The study protocol was registered on PROSPERO (CRD42020182475). The study was conducted and reported according to the PRISMA4 and MOOSE5 guidelines. Studies were included if they: (1) involved pregnant people with βTM or βTI, (2) examined pregnancy complications, maternal and neonatal outcomes, and (3) were controlled trials, cohort studies and case series of more than five pregnancies. Maternal and neonatal outcomes included pre-eclampsia, thromboembolic events, gestational diabetes, maternal iron parameters, gestational age at delivery, mode of delivery and size small for gestational age. Risk of bias was assessed at the individual study level according to the Newcastle-Ottawa Scale for non-randomized studies and according to the Joanna Briggs Institute Checklist for Case Series.
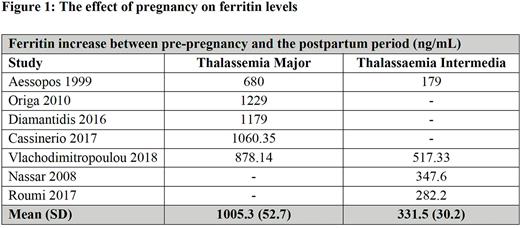
Results Fifteen studies involving 429 participants and 684 pregnancies met inclusion criteria. The studies had a moderate to high risk of bias. Meta-analysis demonstrated an increased risk of thrombosis of 3.7% (95% CI 1.2-75) in βTI compared to βTM (0.9% (95% CI 0.1-2.6)). The risk of heart failure was similar for βTM and βTI [1.6% (95% CI 0.4-3.7) vs. 1.1% (95% CI 0.4-3.7), respectively]. The proportion of patients who developed gestational diabetes (GDM) and pre-eclampsia in βTM was 3.9% (95% CI 1.3-8.0) and 11.3% (95% CI 6.2-15.3), respectively. Maternal mortality was 3.7% (95% CI 0.78-8.6) in βTM and 0% in βTI. Cesarean delivery rate was 83.9% (95% CI 65.2-96.2) in TM and 67% (95%CI 66-77.2) in TI. There were no significant differences in the rate of stillbirth between βTM and βTI [odds ratio (OR) 0.2 (95% CI 0.01-4.25], nor in small for gestational age neonates [OR 2.46, (95% CI 0.5-12.4)]. Similarly, there was no difference in the incidence of preterm birth for βTM and βTI. For βTM, there was a significant increase in red cell (RBC) transfusion requirements during pregnancy, increasing from 102 to 139ml of RBC/kg/year. (p= 0.001; I2 91%, P<0.0001). In pregnancies with βTI, 70% (95% CI 57.7-81.3) required a red cell transfusion during pregnancy. Overall, the pooled mean serum ferritin concentration was significantly increased in βTM (1005 ng/mL) compared to βTI (332 ng/mL) (P<0.0001) (Figure 1). The above iron status deterioration in βTM was also reflected in a significant increase in liver iron concentration from 4.6 (±2.7) to 11.9 (±3.2) mg/g dry weight (p<0.0001) and a significant decrease in myocardial T2* from 36.2±2.5 ms to 31.1 ms±3.4 during pregnancy.
Conclusion While overall pregnancy outcomes for βTM and βTI are favourable, there is a doubling of the risk of stillbirth and increased risk of GDM, pre-eclampsia, preterm birth, and Caesarean section, and as such individuals with these conditions warrant multi-disciplinary pregnancy care in centers with expertise, involving hematology and maternal-fetal medicine. In βTM, there is an escalation of iron loading during pregnancy, as reflected by a rise in serum ferritin levels, increase in liver iron concentration and decrease in myocardial MRI T2*, which may translate to an increased risk of cardiac complications in affected individuals. Future studies characterizing these risks are vital.
Disclosures
Kuo:Pfizer: Consultancy, Research Funding; Bioverativ/Sanofi/Sangamo: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Consultancy; Apellis: Consultancy; bluebird bio: Consultancy; Alexion: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. Malinowski:Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal